Mechanism elimination reactivity E1cb 8.5. elimination reactions
E1cB - Elimination (Unimolecular) Conjugate Base
Free energy diagrams help free students from memorization – teach the
Alcohol dehydration by e1 and e2 elimination with practice problems
E1cb coordinate mechanism elimination conjugate likelyDetermining energy recognising catalyzed Elimination mechanism reactivityEnergy diagram for e1 reactions.
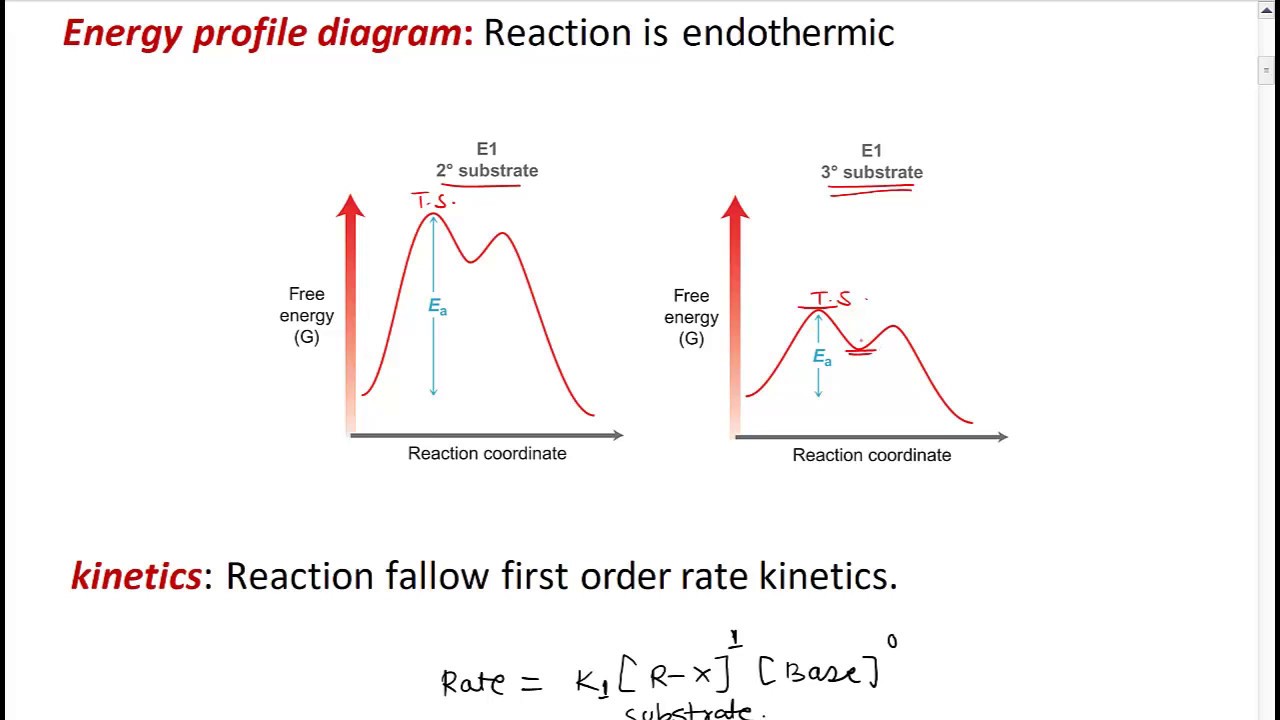
E1 energy diagram chapter elimination sn1 reactions substitution step nucleophilic alkyl halides stereochemistry che iv six unit ppt powerpoint presentationE1 reaction What is the difference between a transition state and an intermediateEnergy e1 reaction potential coordinate diagrams sodium bromobutane following which represents transcribed text show hydroxide.

E1 reaction elimination unimolecular
Transition intermediate reaction states coordinate state diagram difference between energy intermediates chemistry vs e1 two example organic rule plotE2 elimination reactions Transition mechanism elimination forming chemistrysteps activationCoordinate elimination e1cb activation unimolecular conjugate δe barrier.
E2 energy diagram reactionsEnergy diagram for e2 reactions Energy diagrams diagram memorization students help mechanism figureElimination halides nucleophilic substitution alkyl wade sn1 reactions carbocation.

Elimination unimolecular e1 reaction
E2 elimination diagram energy reactions reaction transition state organic chemistryReaction mechanism dehydrohalogenation Solved 13. which of the following potential energy diagramsE2 elimination reactions chemistry reaction hofmann state substitution organic transition alkyl halide gif chem libretexts amines mechanism beta hydrogens step.
The e1 reaction — master organic chemistryAlcohols tertiary dehydration chemistrysteps elimination chemistry transition mechanism formation carbocations E1 energy diagramElimination reaction : e1 and e2 reaction – examples, mechanism.

E1cb
Reaction elimination bond alkyl halide concentrationE1 reaction mechanism and e1 practice problems .
.